Abstract
Background: Heparin induced thrombocytopenia and thrombosis (HIT/HITT) is a disorder characterized by transient hypercoagulability, with increased risk of venous or arterial thrombosis, related to the development of antibodies directed against the heparin-PF4 complex. HIT can occur in any patient receiving heparin but has rarely been described in the setting of high dose melphalan and autologous stem cell transplantation (HDM/SCT) so the true incidence in this population is unknown. Clinical suspicion of HIT in this setting is often clouded by the increased frequency of thrombocytopenia due to stem cell collection or high dose chemotherapy, as well as the increased risk of thrombosis in hematologic malignancies. We report five such cases, with clinically apparent and laboratory confirmed HIT that were diagnosed while undergoing treatment with high dose melphalan and autologous stem cell transplantation.
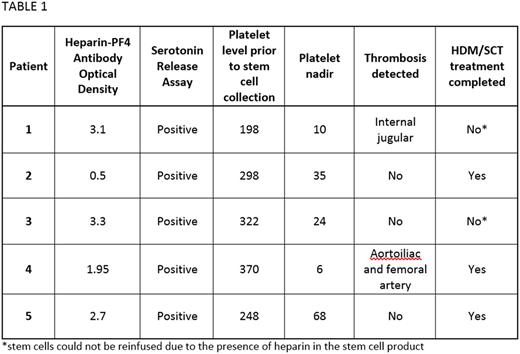
Results: Five patients pursuing treatment with HDM/SCT were diagnosed with HIT during a five year period at our institution. Four patients were being treated for AL amyloidosis and one for multiple myeloma. The average patient age was 64 years (range, 59 to 69), including four males and 1 female. At the time of HIT diagnosis one patient was receiving prophylactic doses of enoxaparin due to a history of line-associated deep vein thrombosis. The other four patients had only been exposed to heparin as part of standard practice for central line placement, line flushes, or stem cell collection. Suspicion of HIT arose in all cases when the patient had delayed platelet recovery during the days following stem cell collection. Only one patient had proceeded with melphalan treatment before HIT was diagnosed. The average platelet nadir was 29, with a range of 6 to 68 x 109/L (Table 1). In two patients the development of thrombosis prompted the work-up for HIT: one with an internal jugular thrombosis and the other with limb threatening aortoiliac thrombosis requiring aortoiliac and femoral artery embolectomy with fasciotomy. All five patients had confirmation of diagnosis with heparin-PF4 antibody (optical density range, 0.5 to 3.3) and a positive serotonin release assay (SRA). Four patients were started on anticoagulation with fondaparinux or argatroban with appropriate platelet recovery in all patients. Three patients proceeded with HDM/SCT, while the remaining two patients received alternative therapies because the previously collected stem cells had been processed with heparin and could not be given to the patients.
Conclusions: Herein we have describe five cases of HIT confirmed by the presence of a heparin-PF4 antibody and SRA testing. In all five cases the cause of thrombocytopenia was initially thought to be related to stem cell collection, which is known to cause a decline in platelets. It is notable that the platelet nadir in these patients, was frequently much lower than the nadir that is typically expected in HIT (50 to 80 x 109/L). Despite the development of thrombosis in two patients and the known increase in mortality in patients with HIT, the long term outcome in each of these patients was favorable. This is potentially attributable to rapid diagnosis, cessation of heparin, and initiation of alternative anticoagulation. In reaction to the initial cases, our institution has changed practices to eliminate heparin in the processing of stem cells so that all patients are able to receive their collected stem cells even in the setting of HIT. These cases highlight the existence of HIT during the process of HDM/SCT and the need for increased diligence in monitoring for unexpected thrombocytopenia or sluggish platelet recovery, in particular immediately after stem cell collection. Clinicians should have a low threshold to send diagnostic testing, begin alternative anticoagulation and postpone high dose chemotherapy in order to prevent life threatening complications.
Sloan: Molecular Templates, Inc.: Consultancy; Stemline Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.